Ideally, the best way to preserve vision is to keep diabetic retinopathy (DR) from developing. DR prevention plays a vital role in thwarting vision loss, as are early detection and treatment.1 However, while medical management and normalization of blood glucose levels are good habits to encourage, people with proliferative diabetic retinopathy (PDR) often require more urgent ocular intervention, including injections or laser, to prevent significant visual consequences.1
Anti-Vascular Endothelial Growth Factor (anti-VEGF)
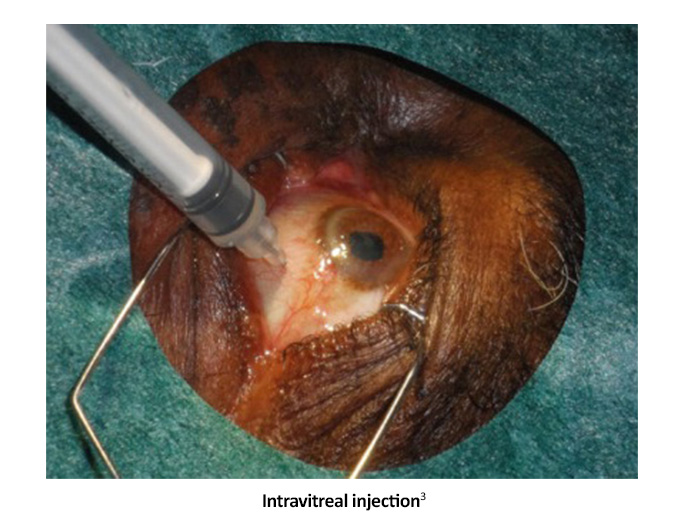
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents are effective in the treatment of center-involved diabetic macular edema (CI-DME) with vision loss, reduce the severity of DR and effectively treat PDR.1
Studies show that people who receive these injections have better average visual acuity, less visual field loss, fewer vitrectomies, and fewer new developments of vision loss due to diabetic macular edema (DME).1
Many patients respond well to anti-VEGF agents, improving 1-3 lines or more on the Snellen vision chart.5 Because of the need for repeat injections to treat both PDR and DME, anti-VEGF agents are best utilized in patients with reliable follow-up and compliance.1
First-generation anti-VEGF agents include ranibizumab and aflibercept, both FDA-approved to treat PDR, or DR of any severity, with or without DME, supported by data from the Protocol 4 S, RISE/RIDE, VIVID/VISTA, and PANORAMA trials. Biosimilar versions of these agents are also available.1,5 Off-label use of bevacizumab is sometimes employed by ophthalmologists as DR therapy.1,5 First-generation anti-VEGFs can require frequent injection intervals, depending on the agent used and the response to treatment, with some patients requiring monthly doses.6
Next-generation anti-VEGF agents include aflibercept 8 mg, faricimab, brolucizumab, and the ranibizumab port delivery system. These agents offer extended dosing intervals after a series of loading doses, which may reduce the burden of frequent first-generation anti-VEGF therapy.6
Faricimab was FDA-approved to treat DME based on the YOSEMITE/RHINE trials. Following a series of loading doses, after which dosing intervals can be extended from 8 – 16 weeks. More than 70% of patients were able to extend dosing intervals to 12 weeks or more.1,5
Brolucizumab has also shown efficacy in DME with more than 50% of patients moving to 12-week dosing intervals through the KITE/KESTRAL trials. These trials linked brolucizumab to a higher incidence (4%) of intraocular inflammation, retinal vasculitis, and retinal vascular occlusion, prompting risk-benefit assessment for patient selection and close treatment monitoring.1
Ranibizumab, delivered via a port delivery system implanted in the sclera, continuously delivers VEGF-A targeting through passive diffusion and is refilled every 6 months. It was approved for treatment of DR and DME based on the PAVILION and PAGODA phase 3 trials, demonstrating non-inferiority to monthly intravitreal ranibizumab.1
Aflibercept 8 mg with a higher molar concentration also received FDA-approval for the treatment of DR and DME.6 In the PHOTON phase 3 trial, 93% of patients maintained intervals of 12 weeks or greater through week 48, with a safety profile similar to aflibercept 2 mg.1